DPPIV/CD26 (EC 3.4.14.5) is an approximately 110 kDa serine exopeptidase that releases Xaa-Pro or Xaa-Ala dipeptides from the N-terminus of oligo- and polypeptides. It regulates immune and endocrine function through the cleavage of multiple chemokines, growth factors, and peptide hormones. Mature human DPPIV consists of a 6 amino acid (aa) cytoplasmic tail, a 22 aa transmembrane segment, and a 738 aa extracellular domain (ECD) that contains the catalytic active site (Ser, Asp, and His charge relay system). Within the ECD, human DPPIV/CD26 shares 84% amino acid sequence identity with mouse and rat DPPIV. DPPIV is expressed as a noncovalent homodimer on the surface of epithelial cells, endothelial cells, and activated lymphocytes, and it can be released by MMP mediated shedding. It cleaves a range of peptide hormones including Glucagon, Glucagon-like Peptides 1 and 2, GIP, GHRH, Procalcitonin, Neuropeptide Y, and Substance P. It is released from adipocytes and induces insulin resistance in adipocytes and skeletal muscle. DPPIV also cleaves many chemokines, resulting in reduced chemotactic activity of CXCL6, 9, 10, 11, 12, and CCL5 but unchanged angiostatic activity of CXCL9 and CXCL10. Cleavage can increase (CCL5), decrease (CXCL12), or have no effect (CCL4) on chemokine blockade of HIV-1 cellular infectivity . In addition, DPPIV cleavage of CCL4 broadens chemokine receptor usage to also include CCR2b. DPPIV serves as a cell entry coreceptor for HIV and coronavirus.
高纯度、高活性、低内毒素、高批间一致性
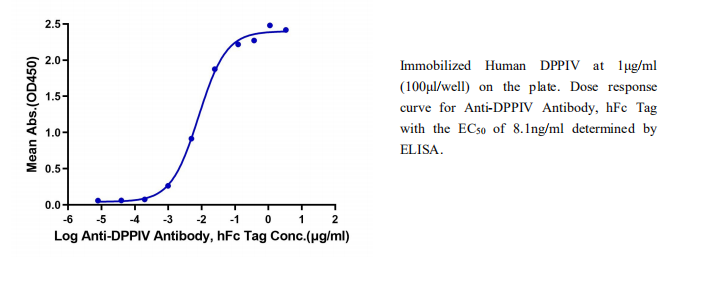
产品数据
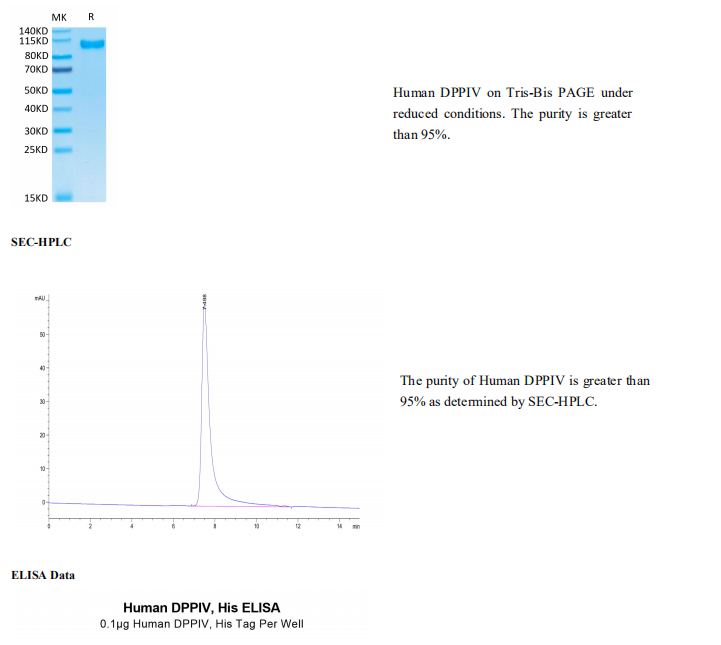
Tris-Bis PAGE
-25 ~ -15℃保存,收到货之后有效期1年。 复溶后, 无菌条件下,-85 ~ -65℃保存,3个月有效期。
