产品说明书
FAQ
COA
已发表文献
Product Description
T7 High Yield RNA Synthesis Kit optimizes the transcription reaction system. The kit can synthesize the single-stranded RNA efficiently by uses T7 RNA polymerase, the linear double-stranded DNA with the T7 promoter sequence as the template, NTPs as the substrate to control the DNA sequence downstream of the promoter. During transcription, modified nucleotides can be added to the substrate to prepare biotin or dye-labeled RNA.
This kit can synthesize long transcripts and short transcripts, RNA can be produced 100-200 μg with 1 μg of DNA template input. The RNA synthesized by transcription can be used for various downstream applications, such as RNA structure and function research, RNase protection, probe hybridization, RNAi, microinjection and in vitro translation.
Contents
|
Contents No. |
Name |
Cat#/Specification |
||
|
10623ES50 (50 T) |
10623ES60 (100 T) |
10623ES70 (500 T) |
||
|
10623-A |
T7 RNA Polymerase Mix |
100 μL |
200 μL |
1 mL |
|
10623-B |
10×Transcription Buffer |
100 μL |
200 μL |
1 mL |
|
10623-C |
ATP(100mM) |
100 μL |
200 μL |
1 mL |
|
10623-D |
CTP(100mM) |
100 μL |
200 μL |
1 mL |
|
10623-E |
GTP(100mM) |
100 μL |
200 μL |
1 mL |
|
10623-F |
UTP(100mM) |
100 μL |
200 μL |
1 mL |
|
10623-G |
Control DNA Template(500ng/μL) |
10 μL |
20 μL |
100μL |
Applications
In vitro RNA synthesis
Shipping and Storage
Dry ice transportation. Store at -20℃, valid for two years.
Notes:
1. Be careful not to mix RNase in the reaction system.
2. Experiment equipment (such as: pipette tip, product tube, etc.) should strictly use RNase Free products.
3. For your safety and health, please wear lab coats and disposable gloves.
4. For research use only!
Synthesis principle
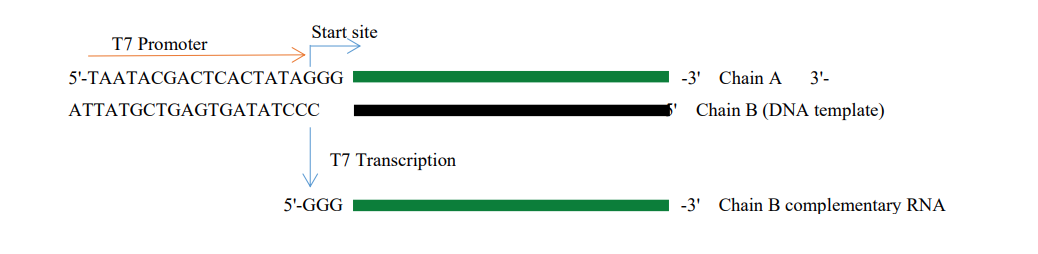
Figure 1: In vitro RNA transcription process
Experimental methods
1. DNA template preparation
Linearized plasmids with double-stranded T7 promoters or PCR amplification products can be used as HifairTM T7 High Yield RNA Synthesis Kit in vitro transcription templates, which can be dissolved in TE buffer or RNase free H2O.
T7 promoter sequence: TAATACGACTCACTATAG*GG (Note: G* is the first base of RNA transcription) A. Plasmid template
Insert the target DNA to the plasmid vector containing the T7 promoter, and then treated with restriction enzymes, purified after completely linearized.
Note: 1. The circular plasmids have no effective termination, RNA products of different lengths will be transcribed. In order to obtain a specific length RNA, the plasmid must be completely linearized.
2. The restriction enzyme selected for plasmid linearization needs to be on the right side of the promoter region, downstream of the inserted DNA fragment, and has no recognition site in the inserted DNA fragment. The restriction enzyme should be capable of forming 5' sticky ends or smooth ends.
3. In order to avoid the influence of protein and salt ions on the system, the plasmid is recommended to be purified when used as a template for in vitro transcription after linearization.
B. PCR product template
The PCR product with T7 promoter can be used as an in vitro transcription template. First, add the T7 promoter sequence (TAATACGA CTCACTATAGGG) to the 5' end of the upstream primer sense strand; next, the T7 promoter DNA template is amplified under the action of high fidelity enzyme; then transcription is performed. PCR products can be used directly as templates without purification, but higher RNA output will be obtained after purification.
Note: 1. The specificity and concentration of the PCR product must be confirmed by electrophoresis when used as a template. Put 2-5 μL of PCR product into the 20 μL reaction system.
2. In order to obtain more high-quality RNA, the PCR product should be recovered by gel and used as a template for in vitro transcription.
2. In vitro RNA transcription
A. Thawing reagents
Centrifuge the T7 RNA Polymerase Mix briefly and place on ice. Thaw 10× Transcription Buffer and ribonucleotides (ATP, CTP, GTP, UTP), mix and centrifuge to the bottom of the tube, place 10× Transcription Buffer at room temperature, and place 4 types of ribonucleotides on ice.
B. Assembly transcription reaction at room temperature
Prepare the reaction system according to the following system:
|
Components |
Volume(μL) |
Final concentration |
|
RNase free H2O |
Up to 20 |
- |
|
10×Transcription Buffer |
2 |
1× |
|
CTP / GTP/ ATP/ UTP (100 mM each) |
2 each |
10 mM each |
|
Template DNA |
1 µg |
- |
|
T7 RNA Polymerase Mix |
2 |
- |
Note: 1. The reaction is configured at room temperature. Since 10× Transcription Buffer contains spermidine, the concentration of spermidine too high will cause DNA template precipitation at low temperature.
2. Short transcript (<100 nt), 2 µg template can be used, transcription time increased to 4-8 hs.
3. For long transcripts (>1000 nt), recommended to use linearized plasmid templates for transcription.
4. Perform the reaction in a PCR machine with the hot lid open to prevent the reaction solution from evaporating for a long time.
5. The reaction product may have a white precipitate. This is free pyrophosphate and magnesium ions produce the magnesium pyrophosphate in the reaction, won’t affect the subsequent experiments. You can add some EDTA to clear it. If the addition of EDTA affects subsequent experiments, the supernatant can also be recovered by centrifugation.
6. The reagents and containers must without RNase contamination.
C. Incubate at 37°C for 2 hours
Mix the above reaction solution, briefly centrifuge to the bottom of the tube, and incubate at 37°C for 2 hs. If the transcript length is less than 100 nt, increase the reaction time to 4-8 hs.
D. DNase I treatment (optional)
After the reaction is complete, add 2 μL of DNase I (RNase free) to each tube and incubate at 37°C for 15 mins to remove the template DNA.
3. Product purification
A. RNA Cleaner magnetic bead
Take out RNA clean beads from 4°C in advance, equilibrate to room temperature (about 30 minutes), dilute the transcription product to 50 μL with RNase free H2O.
①Invert or vortex to mix the magnetic beads thoroughly, add 2× magnetic beads (100 μL) to the RNA sample (50 μL), pipette 6 times to mix thoroughly. Incubate for 5 min at room temperature, allow RNA to bind to the magnetic beads.
②Place the sample on the magnetic stand for 5 mins. After the solution is clear, remove the supernatant carefully.
③Keep the sample on the magnetic stand, add 200 μL of freshly prepared 80% ethanol to rinse the magnetic beads, incubate at room temperature for 30 s, carefully remove the supernatant. Repeat this operation once again.
(Note: The 80% ethanol used for rinsing needs to be freshly prepared with RNase free H2O to prevent the introduction of RNase enzyme from causing RNA degradation.)
④Keep the sample on the magnetic stand, open the lid for 5 mins to dry the beads.
(Note: Avoid excessive drying. If the magnetic beads are cracked, it means that the magnetic beads are too dry and the elution efficiency of RNA will be reduced).
⑤Remove the sample from the magnetic stand, add 22 μL RNase free H2O, pipette 6 times to mix well, incubate for 5 mins at room temperature.
⑥Place the sample on the magnetic stand for 5 mins. After the solution clarifies, carefully transfer 20 μL of the supernatant to a new RNase free PCR tube.
Note: Leave 2-3 μL of liquid when transferring the supernatant, not attract the magnetic beads and affect the subsequent experiments. The obtained RNA is extremely unstable, please proceed to the next step as soon as possible. If you want to save, please store at -80°C.)
B. Phenol/chloroform
①Add 115μL RNase free H2O and 15 μL 3M sodium acetate (pH 5.2) to 20 μL reaction mixture, mix well.
②Extract once with an equal volume of phenol/chloroform (1:1), and then extract twice with an equal volume of chloroform. Collect the supernatant and transfer it to a new RNase free EP tube.
③Add 2 volumes of absolute ethanol to precipitate RNA. Mixing uniformly and place it at -20°C for at least 30 mins, centrifuge at 4°C for 15 mins at maximum speed, and collect the precipitate.
④Add 500 μL of ice-cold 70% ethanol to wash the RNA pellet.
⑤Dissolve the RNA pellet with 20 μL RNase free H2O. Store the purified RNA solution at -80°C.
C. Lithium chloride precipitation
The lithium chloride precipitation method demands the RNA length must be greater than 300 nt, and the concentration must not be less than 100 ng/μL.
①Add 30 μL RNase free H2O and 30 μL 7.5M lithium chloride to 20 μL reaction mixture.
②After mixing uniformly, place it at -20°C for at least 30 mins, centrifuge at 4°C for 15 mins at maximum speed, and collect the precipitate.
③Add 500 μL of ice-cold 70% ethanol to wash the RNA pellet.
④Dissolve the RNA pellet with 20 μL RNase free H2O. The purified RNA solution is stored at -80°C.
D. Column purification
Before purification, add 80μL RNase free H2O to dilute the product to 100 μL, and then follow the column purification instructions for purification.
4. RNA quantification
A. Ultraviolet absorption
B. Dye method
Use RiboGreen dye to quantify RNA, free nucleotides won’t affect quantification, purified or unpurified RNA in reaction products can be accurately quantified.
5. RNA size and quality detection
A. Agarose electrophoresis
In order to determine the size, integrity and quality of RNA, agarose gel electrophoresis or polyacrylamide gel electrophoresis is required for detection.
B. Agilent 2100 Bioanalyzer detection
Agilent 2100 Bioanalyzer can be used to evaluate the integrity and quality of RNA. It only requires a small amount of RNA for analysis. High-quality RNA should show obvious and sharp peaks on the electrogram.
Frequently Asked Questions:
1. Low transcript yield
The quality of template is closely related to the yield. The yield of experimental group is significantly lower than the control group. The possible reasons are: ① the experimental template contains inhibitory components; ② The template has something wrong.
Suggestions: ① Re-purify the template; ② Determine the template quantification and its integrity; ③ Extend the reaction time; ④ Increase the amount of template input; ⑤ Try other promoters and RNA polymerases.
2. Low yield of short transcripts
Short transcription initiation fragment will inhibit the reaction. When the transcription product is less than 100 nt, extending the reaction time to 4-8 hs or increasing the amount of template to 2 μg will increase RNA yield.
3. RNA transcription length is greater than expected
If the electrophoresis shows that the product band is larger than the expected size, the possible reasons: ①The plasmid template may not be completely linearized; ②The 3' end of the sense strand has a prominent structure; ③The RNA has a secondary structure that is not completely denatured.
Suggestions: ①Check whether the template is completely linearized, and if necessary, perform additional linearization; ②Select a suitable restriction enzyme to avoid 3' overhangs, or use Klenow Fragment /T4 DNA polymerase to complete the transcription before proceeding; ③Use denatured gel to detect RNA products.
4. RNA transcription length is less than expected
If the electrophoresis shows that the product band is smaller than the expected size, the possible reasons: ①The template contains a termination sequence similar to T7 RNA polymerase; ②The GC content in the template is high.
Suggestions: ①Lower the reaction temperature (for example, 30°C). Sometimes lowering the temperature can increase the transcription length, but it will reduce the yield. Or try different RNA polymerases for transcription; ②If the template GC content is high, use 42℃ to transcript, or add SSB to increase the yield and transcription length.
5. Electrophoresis tailing of transcription products
There is tailing phenomenon during electrophoresis. Possible reasons: ①Contaminated by RNase during experimental operation; ②Contaminated DNA template by RNase.
Suggestions: ①Use RNase-free pipette tips and EP tubes, wear disposable latex gloves and masks, and all reagents are prepared with RNase free H2O. ②Re-purify the template DNA.
HB220514