LysoTracker®系列探针是对活细胞中的酸性区室进行选择性染色的一类荧光染料,该类探针具有几大重要的特点,1)选择性标记酸性细胞器;2)纳摩尔级(nM)浓度即可有效标记活细胞;3)具有多色探针提供,可根据情况对样品进行多标实验。LysoTracker®结构上由一个荧光基团和相连的弱碱基构成,可自由穿过细胞膜,一般聚集在球形细胞器上,适用于观察溶酶体内部生物合成及相关发病机理。Lysotracker中性pH下仅仅发生部分质子化,因此该探针标记细胞器的原理可能与其完全质子化并滞留在细胞器膜上有关。
本品LysoTracker® Red DND-99为红色荧光标记的溶酶体探针,具有577/590 nm的最大激发/发射波长。本品以溶于无水DMSO的1mM储存液形式提供。
| CAS号(CAS NO.) | N/A |
| 分子式(Formula) | C20H24BF2N5O |
| 分子量(Molecular Weight) | 399.2493 |
| Ex/Em(nm) | 577/590 |
| 外观(Appearance) | 紫色溶液 |
| 结构式(Structure) | 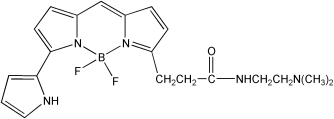 |
室温运输。-20℃避光保存,有效期1年,避免反复冻融。
Q:染色时,细胞的状态?
A:这四种探针是对活细胞中的酸性区室进行染色。所以必须要在活细胞状态下染色。
Q:染色完成后,细胞是否能固定后再检测荧光信号?
A:固定后会破坏溶酶体的酸性环境从而会造成荧光减弱甚至消失的情况,染色前后均不建议固定。
Q:可以和二抗一起孵育吗?
A:不可以,孵育二抗必须要透化处理,而透化会破坏溶酶体的酸性环境,影响染色效果。
Q:这个探针可以染自噬溶酶体吗?可以染色其他酸性细胞器吗?
A:自噬溶酶体和正常的溶酶体在形态结构上有较大差异,该探针主要是针对常规的溶酶体染色,对于自噬溶酶体的染色效果不是很确定,可以参考一些使用过该产品发表的文献资料确定可以染色其他酸性细胞器,建议极低浓度下才能优先选择染色酸性溶酶体。
Q:植物细胞和组织适用吗?
A:理论上可以,一般植物细胞或者组织要制成原生质体。
Q:染色后可以放置一夜,再荧光检测吗?
A:建议染色完立即检测荧光,时间过长,荧光信号会逐渐减弱。
Q:用细胞培养基配成工作液以后稳定吗?可以存放多久?
A:工作液是需要现配现用的,不建议保存。
Q:检测仪器?
A:荧光显微镜 共聚焦显微镜 酶标仪 流式细胞仪。
Q:如何做到同时染色溶酶体和细胞核的?
A:建议用Hoechst 33258/ Hoechst 33342 染色细胞核,这个染料可以直接染活细胞不需要固定操作。
Q:保质期多久?
A:-20℃避光保存,半年有效。
Q:荧光信号较弱?
A:大致有 3 个原因: 溶酶体探针浓度过低,建议适当增大浓度; 染色时间过短,建议延长染色时间; 染色完之后,放置过长时间才检测信号;建议染色完立刻检测。
Q:没有荧光信号?
A:大致有 3 个原因: 先用多聚甲醛 乙醛等固定细胞,破坏了溶酶体的酸性环境。 染色完后,又用多聚甲醛固定细胞,或者透化细胞,破坏了溶酶体的酸性环境;染色前后均不建议固定细胞;
- 染色完到上机检测这一段时间过长,并且没有避光处理。建议避光染色,染色完立即检测荧光信号。
Q:在活细胞状态下,用 50 M 探针染色 50 min,然后发现除了溶酶体,细胞质中也有荧光信号, 这主要是什么原因导致?
A:大致有一下 2 个原因:
- 溶酶体探针浓度过大,建议适当降低浓度;
- 溶酶体探针染色时间过长,建议适当缩短时间。
[1] Wang Y, Chen J, Duan R, et al. High-Z-Sensitized Radiotherapy Synergizes with the Intervention of the Pentose Phosphate Pathway for In Situ Tumor Vaccination. Adv Mater. 2022;34(13):e2109726. doi:10.1002/adma.202109726(IF:30.849)
[2] Feng L, Dou C, Xia Y, et al. Neutrophil-like Cell-Membrane-Coated Nanozyme Therapy for Ischemic Brain Damage and Long-Term Neurological Functional Recovery. ACS Nano. 2021;15(2):2263-2280. doi:10.1021/acsnano.0c07973(IF:15.881)
[3] Wan SS, Zhang L, Zhang XZ. An ATP-Regulated Ion Transport Nanosystem for Homeostatic Perturbation Therapy and Sensitizing Photodynamic Therapy by Autophagy Inhibition of Tumors. ACS Cent Sci. 2019;5(2):327-340. doi:10.1021/acscentsci.8b00822(IF:12.837)
[4] Liang H, Peng B, Dong C, et al. Cationic nanoparticle as an inhibitor of cell-free DNA-induced inflammation. Nat Commun. 2018;9(1):4291. Published 2018 Oct 16. doi:10.1038/s41467-018-06603-5(IF:12.353)
[5] Gong Y, Li Z, Zou S, et al. Vangl2 limits chaperone-mediated autophagy to balance osteogenic differentiation in mesenchymal stem cells. Dev Cell. 2021;56(14):2103-2120.e9. doi:10.1016/j.devcel.2021.06.011(IF:12.270)
[6] Niu B, Zhou Y, Liao K, et al. "Pincer movement": Reversing cisplatin resistance based on simultaneous glutathione depletion and glutathione S-transferases inhibition by redox-responsive degradable organosilica hybrid nanoparticles. Acta Pharm Sin B. 2022;12(4):2074-2088. doi:10.1016/j.apsb.2021.10.013(IF:11.614)
[7] Li Z, Wu M, Liu S, et al. Apoptotic vesicles activate autophagy in recipient cells to induce angiogenesis and dental pulp regeneration [published online ahead of print, 2022 May 10]. Mol Ther. 2022;S1525-0016(22)00304-5. doi:10.1016/j.ymthe.2022.05.006(IF:11.454)
[8] Wang H, Shi W, Zeng D, et al. pH-activated, mitochondria-targeted, and redox-responsive delivery of paclitaxel nanomicelles to overcome drug resistance and suppress metastasis in lung cancer. J Nanobiotechnology. 2021;19(1):152. Published 2021 May 22. doi:10.1186/s12951-021-00895-4(IF:10.435)
[9] Han C, Xu X, Zhang C, et al. Cytochrome c light-up graphene oxide nanosensor for the targeted self-monitoring of mitochondria-mediated tumor cell death [published online ahead of print, 2020 Nov 5]. Biosens Bioelectron. 2020;173:112791. doi:10.1016/j.bios.2020.112791(IF:10.257)
[10] Liu H, Liu S, Qiu X, et al. Donor MSCs release apoptotic bodies to improve myocardial infarction via autophagy regulation in recipient cells. Autophagy. 2020;16(12):2140-2155. doi:10.1080/15548627.2020.1717128(IF:9.770)
[11] Wu D, Zhu X, Ao J, Song E, Song Y. Delivery of Ultrasmall Nanoparticles to the Cytosolic Compartment of Pyroptotic J774A.1 Macrophages via GSDMDNterm Membrane Pores. ACS Appl Mater Interfaces. 2021;13(43):50823-50835. doi:10.1021/acsami.1c17382(IF:9.229)
[12] Zhao Q, Li J, Wu B, et al. Smart Biomimetic Nanocomposites Mediate Mitochondrial Outcome through Aerobic Glycolysis Reprogramming: A Promising Treatment for Lymphoma. ACS Appl Mater Interfaces. 2020;12(20):22687-22701. doi:10.1021/acsami.0c05763(IF:9.229)
[13] Zhu Q, Liu Z, Wang Y, Song E, Song Y. Endoplasmic reticulum stress manipulates autophagic response that antagonizes polybrominated diphenyl ethers quinone induced cytotoxicity in microglial BV2 cells. J Hazard Mater. 2021;411:124958. doi:10.1016/j.jhazmat.2020.124958(IF:9.038)
[14] Peng J, Pan J, Wang H, Mo J, Lan L, Peng Y. Morphine-induced microglial immunosuppression via activation of insufficient mitophagy regulated by NLRX1. J Neuroinflammation. 2022;19(1):87. Published 2022 Apr 12. doi:10.1186/s12974-022-02453-7(IF:8.322)
[15] Wu R, Mei X, Ye Y, et al. Zn(II)-curcumin solid dispersion impairs hepatocellular carcinoma growth and enhances chemotherapy by modulating gut microbiota-mediated zinc homeostasis. Pharmacol Res. 2019;150:104454. doi:10.1016/j.phrs.2019.104454(IF:7.658)
[16] Liu ZH, Xu HL, Han GW, et al. Self-Assembling Nanovaccine Enhances Protective Efficacy Against CSFV in Pigs. Front Immunol. 2021;12:689187. Published 2021 Jul 21. doi:10.3389/fimmu.2021.689187(IF:7.561)
[17] Liu Z, Lv X, Xu L, et al. Zinc oxide nanoparticles effectively regulate autophagic cell death by activating autophagosome formation and interfering with their maturation. Part Fibre Toxicol. 2020;17(1):46. Published 2020 Sep 18. doi:10.1186/s12989-020-00379-7(IF:7.546)
[18] Xing Y, Zhang J, Chen F, Liu J, Cai K. Mesoporous polydopamine nanoparticles with co-delivery function for overcoming multidrug resistance via synergistic chemo-photothermal therapy. Nanoscale. 2017;9(25):8781-8790. doi:10.1039/c7nr01857f(IF:7.367)
[19] Huang HJ, Cui JR, Xia X, et al. Salivary DNase II from Laodelphax striatellus acts as an effector that suppresses plant defence. New Phytol. 2019;224(2):860-874. doi:10.1111/nph.15792(IF:7.299)
[20] Gao Y, Gao J, Mu G, et al. Selectively enhancing radiosensitivity of cancer cells via in situ enzyme-instructed peptide self-assembly. Acta Pharm Sin B. 2020;10(12):2374-2383. doi:10.1016/j.apsb.2020.07.022(IF:7.097)
[21] Liu ZH, Xu HL, Han GW, et al. A self-assembling nanoparticle: Implications for the development of thermostable vaccine candidates. Int J Biol Macromol. 2021;183:2162-2173. doi:10.1016/j.ijbiomac.2021.06.024(IF:6.953)
[22] Yang N, Tang Q, Qin W, et al. Treatment of obesity-related inflammation with a novel synthetic pentacyclic oleanane triterpenoids via modulation of macrophage polarization. EBioMedicine. 2019;45:473-486. doi:10.1016/j.ebiom.2019.06.053(IF:6.680)
[23] Wang Z, Wang L, Prabhakar N, et al. CaP coated mesoporous polydopamine nanoparticles with responsive membrane permeation ability for combined photothermal and siRNA therapy. Acta Biomater. 2019;86:416-428. doi:10.1016/j.actbio.2019.01.002(IF:6.638)
[24] Wang H, Zhang F, Wen H, et al. Tumor- and mitochondria-targeted nanoparticles eradicate drug resistant lung cancer through mitochondrial pathway of apoptosis. J Nanobiotechnology. 2020;18(1):8. Published 2020 Jan 9. doi:10.1186/s12951-019-0562-3(IF:6.518)
[25] Zhai L, Luo C, Gao H, Du S, Shi J, Wang F. A Dual pH-Responsive DOX-Encapsulated Liposome Combined with Glucose Administration Enhanced Therapeutic Efficacy of Chemotherapy for Cancer. Int J Nanomedicine. 2021;16:3185-3199. Published 2021 May 10. doi:10.2147/IJN.S303874(IF:6.400)
[26] Chen S, Dong G, Wu S, Liu N, Zhang W, Sheng C. Novel fluorescent probes of 10-hydroxyevodiamine: autophagy and apoptosis-inducing anticancer mechanisms. Acta Pharm Sin B. 2019;9(1):144-156. doi:10.1016/j.apsb.2018.08.003(IF:6.014)
[27] Xu Q, Zhang T, Wang Q, et al. Uniformly sized iron oxide nanoparticles for efficient gene delivery to mesenchymal stem cells. Int J Pharm. 2018;552(1-2):443-452. doi:10.1016/j.ijpharm.2018.10.023(IF:5.875)
[28] Mamat M, Wang X, Wu L, et al. CaO2/Fe3O4 nanocomposites for oxygen-independent generation of radicals and cancer therapy. Colloids Surf B Biointerfaces. 2021;204:111803. doi:10.1016/j.colsurfb.2021.111803(IF:5.268)
[29] Wu Y , Zhang X , Li H , et al. A core/shell stabilized polysaccharide-based nanoparticle with intracellular environment-sensitive drug delivery for breast cancer therapy. J Mater Chem B. 2018;6(41):6646-6659. doi:10.1039/c8tb00633d(IF:4.776)
[30] Yang P, Zhang S, Wang K, Qi H. Synthesis of pH-responsive cyclometalated iridium(III) complex and its application in the selective killing of cancerous cells. Dalton Trans. 2021;50(46):17338-17345. Published 2021 Nov 30. doi:10.1039/d1dt03042f(IF:4.390)
[31] Shen Y, Zhang J, Hao W, et al. Copolymer micelles function as pH-responsive nanocarriers to enhance the cytotoxicity of a HER2 aptamer in HER2-positive breast cancer cells. Int J Nanomedicine. 2018;13:537-553. Published 2018 Jan 25. doi:10.2147/IJN.S149942(IF:4.370)
[32] Li B, Wu J, Bao J, et al. Activation of α7nACh receptor protects against acute pancreatitis through enhancing TFEB-regulated autophagy. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165971. doi:10.1016/j.bbadis.2020.165971(IF:4.352)
[33] Li B, Wu J, Bao J, et al. Activation of α7nACh receptor protects against acute pancreatitis through enhancing TFEB-regulated autophagy. Biochim Biophys Acta Mol Basis Dis. 2020;1866(12):165971. doi:10.1016/j.bbadis.2020.165971(IF:4.352)
[34] Shen S, Li B, Dai J, et al. BRD4 Inhibition Protects Against Acute Pancreatitis Through Restoring Impaired Autophagic Flux. Front Pharmacol. 2020;11:618. Published 2020 May 8. doi:10.3389/fphar.2020.00618(IF:4.225)
[35] Xu D, Su Y, Xu Q, Huang T, Chen Z, Zhang T. Uniform iron oxide nanoparticles reduce the required amount of polyethylenimine in the gene delivery to mesenchymal stem cells. Nanotechnology. 2021;33(12):10.1088/1361-6528/ac4066. Published 2021 Dec 24. doi:10.1088/1361-6528/ac4066(IF:3.874)
[36] Zhang H, Li M, Zhu X, Zhang Z, Huang H, Hou L. Artemisinin co-delivery system based on manganese oxide for precise diagnosis and treatment of breast cancer. Nanotechnology. 2021;32(32):10.1088/1361-6528/abfc6f. Published 2021 May 17. doi:10.1088/1361-6528/abfc6f(IF:3.874)
[37] Li Z, Jiang T, Lu Q, et al. Berberine attenuated the cytotoxicity induced by t-BHP via inhibiting oxidative stress and mitochondria dysfunction in PC-12 cells. Cell Mol Neurobiol. 2020;40(4):587-602. doi:10.1007/s10571-019-00756-7(IF:3.811)
[38] Ye X, Han X, Li B, et al. Dopamine D2 receptor activator quinpirole protects against trypsinogen activation during acute pancreatitis via upregulating HSP70. Am J Physiol Gastrointest Liver Physiol. 2020;318(6):G1000-G1012. doi:10.1152/ajpgi.00354.2019(IF:3.725)
[39] Lu D, An Y, Feng S, et al. Imidazole-Bearing Polymeric Micelles for Enhanced Cellular Uptake, Rapid Endosomal Escape, and On-demand Cargo Release. AAPS PharmSciTech. 2018;19(6):2610-2619. doi:10.1208/s12249-018-1092-2(IF:2.666)